The Oxford/AstraZeneca vaccine has become the second coronavirus jab to be approved for UK use.
It has been given the go-ahead by the Medicines and Healthcare products Regulatory Agency (MHRA).
The vaccine, codenamed AZD1222 or ChAdOx1 nCoV-19, was developed at Oxford University with support from the British-Swedish pharmaceutical giant AstraZeneca.
Results from clinical trials showed that it is up to 90% effective in preventing COVID-19, almost matching the protection by the rival Pfizer/BioNTech and Moderna jabs.
Health Secretary Matt Hancock told Sky News the Oxford vaccine will start to be rolled out from 4 January and that it will help “accelerate” vaccinating the nation.
He said: “It’s very good news for accelerating the vaccine rollout. It brings forward the day we can get our lives back to normal.
“The vaccine is our way out of the pandemic.”
Although, he refused to put a figure on exactly how many people could be vaccinated in the new year.
But Mr Hancock did say the jab, which can be stored more easily than the Pfizer vaccine, means GPs and care homes will have better access to the vaccine.
Prime Minister Boris Johnson said: “It is truly fantastic news – and a triumph for British science – that the vaccine has been approved for use. We will now move to vaccinate as many people as quickly as possible.”
A Department of Health and Social Care spokesperson said: “The government has today accepted the recommendation from the Medicines and Healthcare products Regulatory Agency (MHRA) to authorise Oxford University/AstraZeneca’s COVID-19 vaccine for use.
“This follows rigorous clinical trials and a thorough analysis of the data by experts at the MHRA, which has concluded that the vaccine has met its strict standards of safety, quality and effectiveness.
“Now the NHS will begin putting their extensive preparations into action to roll out the Oxford University/AstraZeneca vaccine.”
The government said the vaccine rollout will change slightly to focus on giving as many at-risk people as possible the initial vaccine dose.
People receiving the Oxford vaccine or the one from Pfizer/BioNTech, which is also being rolled out, will now receive their first dose of the vaccine followed by a second dose up to 12 weeks later.
This was confirmed by Mr Hancock who said the second dose is important to help boost “long-term coverage”.
A statement from the Department of Health said: “The JCVI has advised the priority should be to give as many people in at-risk groups their first dose, rather than providing the required two doses in as short a time as possible.
“Everyone will still receive their second dose and this will be within 12 weeks of their first. The second dose completes the course and is important for longer term protection.
“From today the NHS across the UK will prioritise giving the first dose of the vaccine to those in the most high-risk groups. With two vaccines now approved, we will be able to vaccinate a greater number of people who are at highest risk, protecting them from the disease and reducing mortality and hospitalisation.”
Pascal Soriot, chief executive of AstraZeneca, said: “Today is an important day for millions of people in the UK who will get access to this new vaccine.
“It has been shown to be effective, well-tolerated, simple to administer and is supplied by AstraZeneca at no profit.”
Professor Andrew Pollard, director of the Oxford Vaccine Group who led the clinical trial, said: “Though this is just the beginning, we will start to get ahead of the pandemic, protect health and economies when the vulnerable are vaccinated everywhere, as many as possible as soon possible.”
It comes amid increasing strain on hospitals in England, where the number of coronavirus patients is at its highest ever level during the pandemic.
Mr Hancock is due to announce any changes to tier areas in a statement to the Commons on Wednesday afternoon.
Independent analysis published in The Lancet previously confirmed the Oxford/AstraZeneca vaccine is safe and effective after looking into phase 3 data of a vaccine trial.
Once regarded as the frontrunner in the COVID vaccine race, the British team were overtaken by US drugmaker Pfizer with a success rate of 95%, with that jab already being rolled out in the UK.
While the vast majority of the 100 million doses of the drug ordered by the UK from AstraZeneca will be made in the UK, giving the Oxford vaccine a considerable advantage over its rivals, the initial rollout will use doses manufactured in Europe.
AstraZeneca said it aimed to supply millions of doses in the first quarter of next year.
Unlike the Pfizer vaccine, the Oxford vaccine does not need to be stored at ultra-low temperatures.
Like most other vaccines, it will need to be sent to vaccination centres in refrigerated vans or cool boxes and kept in a special vaccine fridge between 2C to 8C, and protected from light.
It is also the cheapest of all the COVID vaccines, costing “the same as a cup of coffee”, as Sky News understands it will be a little under £3 per dose, with one and a half or two doses required.
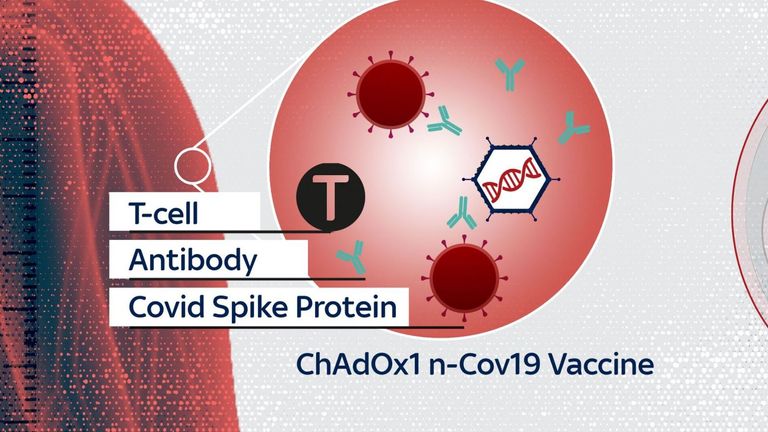
The vaccine is based on an adenovirus, which causes the common cold in chimpanzees.
The virus is modified so it can’t cause disease in humans. It also carries an extra gene from the coronavirus.
Inside the human body, the gene makes the spike protein that coats the shell of the coronavirus, stimulating an immune response that protects against infection.
For one course of dosing – where people were given a half dose of the Oxford vaccine, followed by a full measure at least a month after – there was an efficacy rate of 90%.
That fell to an efficacy of 62% when two full doses were given at least a month apart.
A total of 2,741 people were on the course that proved 90% effective, while 8,895 were given two full doses.
When all the results are tabulated, the average efficacy of the vaccine worked out to 70%.