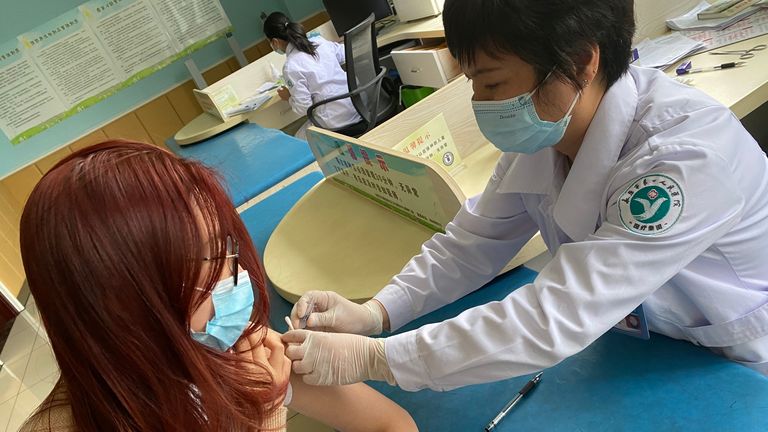
When Evelyn Wu heard she could get a COVID-19 vaccine, she jumped at the chance – even though it hasn’t been scientifically proven.
“I felt excited actually,” she told Sky News. “It’s just like a normal vaccine.”
The 20-year-old is an economics student at the University of Birmingham and wants to return there in January.
So, as soon as she heard a coronavirus vaccine was available, she went to the hospital in Yongkang, eastern China, last Monday to register. Two days later, she had her appointment.
“I needed to sign some contract. It has the details about COVID-19. And it told me that it’s very safe, even though it’s only stage 3.”
Ms Wu signed the form acknowledging that she might experience some mild symptoms and promptly received the first of two doses of the vaccine. In total she will pay 456 RMB – about £52.
The vaccine is made by Sinovac, a Beijing-based biotech company. The company is still carrying out late stage trials in Brazil, Turkey and Indonesia, and has said it could publish preliminary phase 3 trial data in November.
That means it hasn’t met the typical safety and efficacy standard for vaccine development – but China has still cleared it for emergency use, saying it had support from the WHO.
“Yes, I’m a little worried about [it being] experimental stage 3,” Ms Wu told Sky News.
“And I think I was the one who was the test subject, the one who was treated like a little mouse.”
Ms Wu said she felt no side effects, apart from a little sleepiness. The hospital will not monitor her directly but she has been told to visit immediately if she develops any symptoms.
And for Ms Wu, government approval is more important than scientific.
“I trust China and I think it’s totally safe to get vaccinated. I trust the government.”
Others have been less trusting, according to Ms Wu. She says that, last month, the government asked doctors and teachers whether they would like to take the vaccine too.
“But some doctors and some teachers refused to make the vaccination. They think it’s dangerous because they think they are being tested, ” she told Sky News.
“They don’t want to be the volunteer to get the experimental vaccine.”
Starting in July, thousands of employees from Chinese state-owned enterprises have already received the vaccine ahead of foreign travel.
But this new campaign is extending that offer to the general public, in a handful of cities and towns, with some restrictions. Volunteers must be aged 18-59 and be a local resident.
Health authorities in Zhejiang province, in east China, have published notices advertising the vaccine. Priority is given to medical workers, people working at border and quarantine centres, public sector workers travelling to mid to high risk COVID-19 areas, and then those who want to take the vaccine. Ms Wu had to show her university identity documents as proof of her intention to travel.
It is not quite a full roll-out yet. One health clinic in Jiaxing, a city in the same province which has advertised the vaccine, told Sky News it was waiting for doses to arrive but that people could sign up in the meantime.
But taking an experimental vaccine brings risk. Phase 3 trials of competing UK and US vaccines, by AstraZeneca and Johnson & Johnson, were temporarily halted after participants fell seriously ill. No such incidents have been publicly recorded by Chinese companies.
And Ms Wu isn’t put off by those interruptions to testing. “I don’t think we’ll have the same problem,” she said. “Because they’re totally different experiments I think. China uses different ways to treat the pandemic.”
She will have her second dose of the vaccine in November – the hospital recommended a gap of 14-28 days between doses. On her return to the UK, she says she will still wear a face mask, observe social distancing and wash her hands thoroughly.
But the vaccination has brought her mother, who insisted she escape the UK back in March, some peace of mind.
“She is happy for me to have the vaccination,” she told Sky News. “Because she thinks I am brave. I make an example for others.
“Because there is an old saying, the first one to eat the crabs is the most brave, right?”